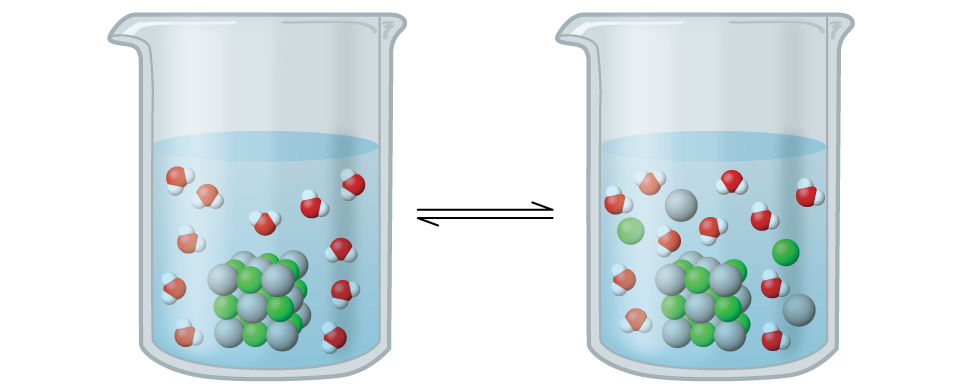
Describe the Formation of an Aqueous Ki Solution
How is a saturated solution in water prepared from a given solute. Qualitative Predictions about Entropy.
15 1 Precipitation And Dissolution Chemistry
The intensity of this pink color is directly related to the concentration of.
. Separation of an unsaturated aqueous solution of potassium chloride into solid KCl and liquid water. Entropy is the randomness of a system. Nonspontaneous spontaneous spontaneous nonspontaneous.
At the molecular level entropy can be described in terms of the possible number of different arrangements of particle positions and. To you it seems obvious that you cannot kill all the cells in step 3 if you need live ones for step 7. Question 13 Determine the empirical formula of an oxide of iron which has 699 iron and 301 dioxygen by mass.
A Ti 3 SiC 2 MAX phase is immersed in CuCl 2 Lewis molten salt at 750 C. 120 g of KI 25 g of KCI 88 g of NaNO_3 and 5 g of KCIO_3. A student prepares four aqueous solutions in 100 g of H_2O at 20 C.
The molar concentration of Br- ions 3 x 0225 M 0675 M 43 A2Y is the strongest electrolyte because it is completely dissociated into ions. This detailed review compiles thorough literature of current research over the last ten years 20062016 and highlights the key findings of adsorption studies that use clay minerals as an adsorbent. Quantitative Descriptions of Solutions A more.
44 a Ionic equation. A2X is the weakest electrolyte because it is the least dissociated of the three substances. Reactions in Aqueous Solution 41 a precipitation b redox c acid-base neutralization 42 FeBr3 contains 3 Br- ions.
The other three people in your group suggest that you pour the Lugols fixative solution into your one and only supply of cells even though you also are supposed to mate LIVING cells later in the lab. A chemical equation that shows all. The authors observed a marked base dependency in the reaction and the requirement for an aqueous co-solvent.
Bc The reaction between Ti 3 SiC 2 and CuCl 2 results in the formation of Ti 3 C 2 T x MXene. I H2O ii CO2 iii CH4 Question 12 Calculate the mass per cent of different elements present in sodium sulphate Na2SO4. Which solution is saturated.
A precipitation reaction is a reaction in which two or more water-_____ ionic compounds react in aqueous solution to form one or more _____ precipitates. After shaking the aqueous phase is separated from the non-polar solvent placed in a test-tube and heated for 20 minutes in boiling water which produces a pink color. Unfortunately you have been cursed with short-sighted lab mates.
Question 14 Calculate the amount of carbon dioxide. I have a question. Question 11 Calculate the molecular mass of the following.
To describe solutions accurately we must describe how much of each component is present Solution Concentration Dilute solutions have a small amount of solute compared to solvent Concentrated solutions have a large amount of solute compared to solvent Tro. CV studies revealed that TEMPO is preferentially oxidized to the corresponding oxoammonium salt E 12 070 V vs AgAgCl in MeCN 149 compared to the carbamate substrate eg for cyclohexenyl N -phenyl carbamate E p2 ox 153 V vs. An _____ ionic equation shows all soluble ionic substances dissociated into ions.
A Molecular Approach 2e 4. Both the atoms and the electrical _____ must be balanced in this type of equation. Clays and their minerals both in its natural and modified forms effectively remove various heavy metals from aqueous solution as extensively discussed in this review.
A general Lewis acidic etching route for preparing MXenes with enhanced electrochemical performance in non-aqueous electrolyte. So you assert yourself. An aqueous solution of TBA reagent is added to the flask and the sample is shaken which causes the polar secondary products to be dissolved in it.
For example suppose 40 g of NaOH or 010 mol of NaOH are dissolved to make 1000 mL of aqueous solution while 365 g of HCl or 010 mol of HCl are dissolved to make another 1000 mL of aqueous solution. The two solutions are mixed in an insulated calorimeter a thermometer is inserted and the calorimeter is covered see Figure 24 Calorimeters for an. In my method of measurements of Moisture in Cosmetics by the distillation.

The Increase In The Solubility Of Iodine In An Aqueous Solution Of Potassium Youtube

Equation For Ki H2o Potassium Iodide Water Youtube

Solved Describe The Formation Of An Aqueous Libr Solution When Solid Libr Dissolves In Water
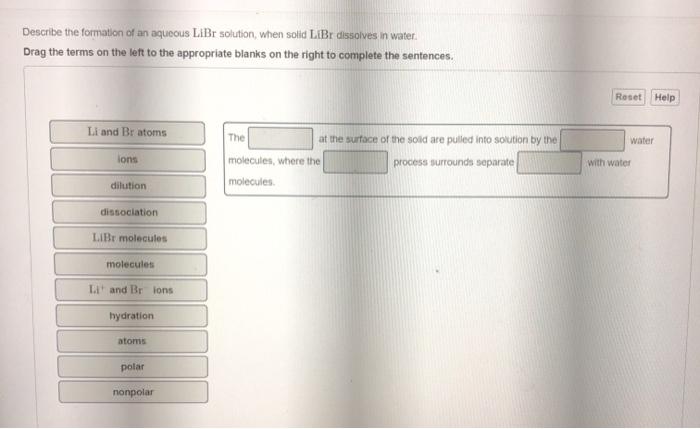
Solved Describe The Formation Of An Aqueous Libr Solution Chegg Com

When Potassium Iodide Solution Is Added To A Solution Of Lead Ii Nitrate In A Test Tube A Prec Youtube

Precipitation Reactions Osmosis

Solved The Electrolysis Of An Aqueous Solution Of Potassium Chegg Com
Reaction Of Chlorine With Potassium Iodide

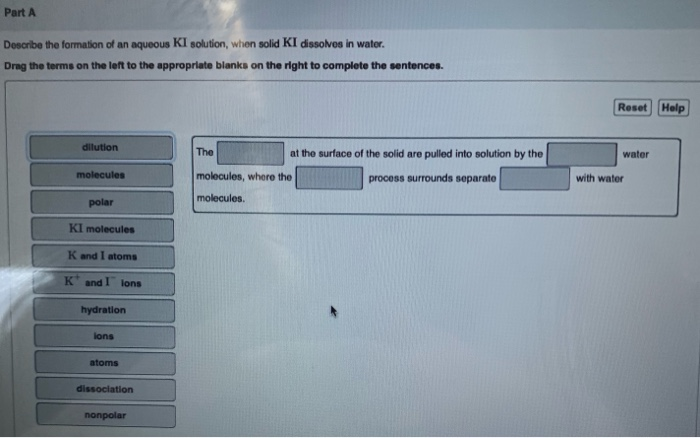
Solved Describe The Formation Of An Aqueous Ki Solution When Solid Ki Dissolves In Water
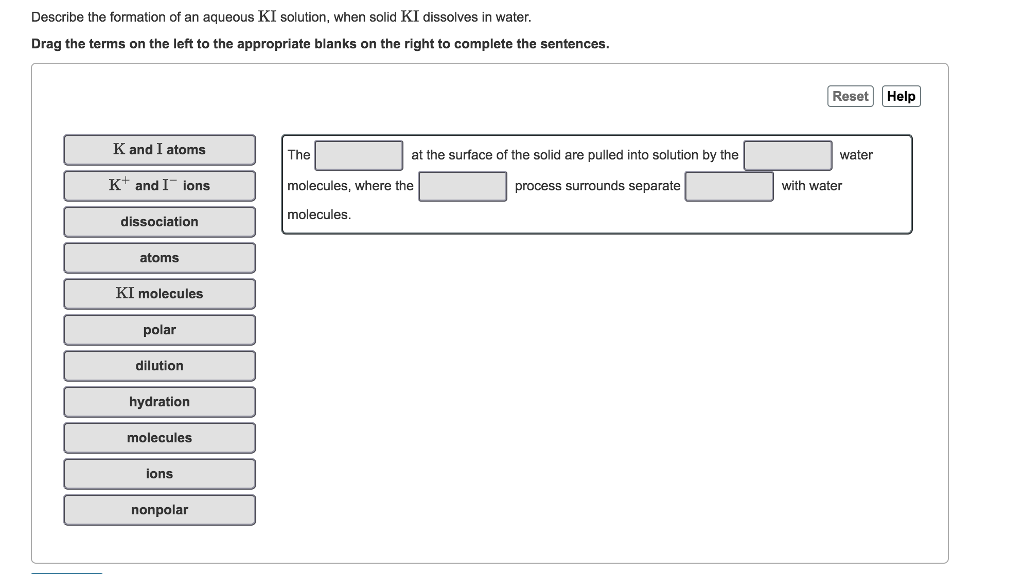
Solved Describe The Formation Of An Aqueous Ki Solution Chegg Com

Using Sodium Hydroxide Solution To Identify Metal Ions Video Lesson Transcript Study Com

Aqueous Solution Chemistry Youtube
Reaction Of Chlorine With Potassium Iodide

Solved Describe The Formation Of An Aqueous Ki Solution When Solid Ki Dissolves In Water
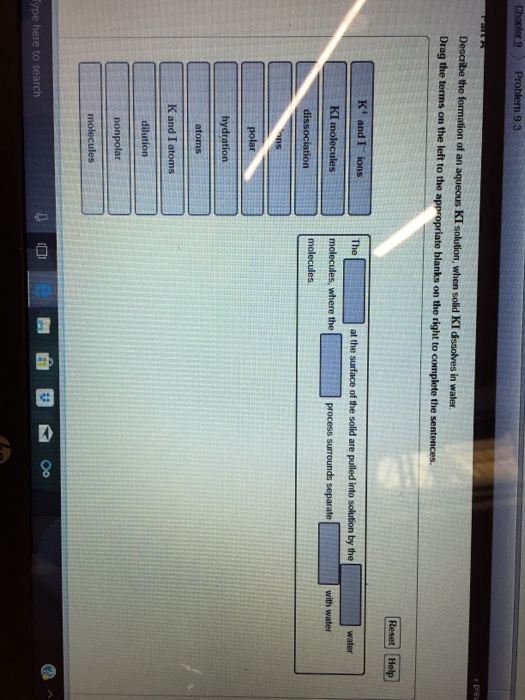
Solved Describe The Formation Of An Aqueous Ki Solution Chegg Com

Solved Part A Describe The Formation Of An Aqueous Ki Chegg Com

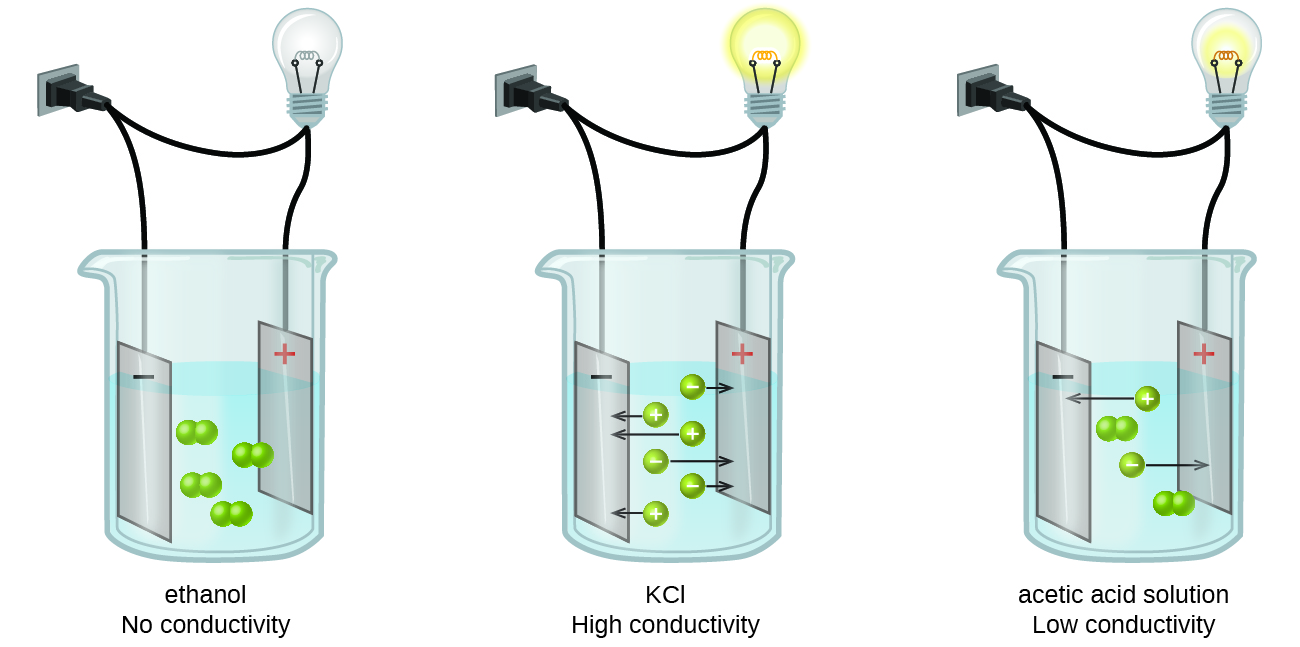
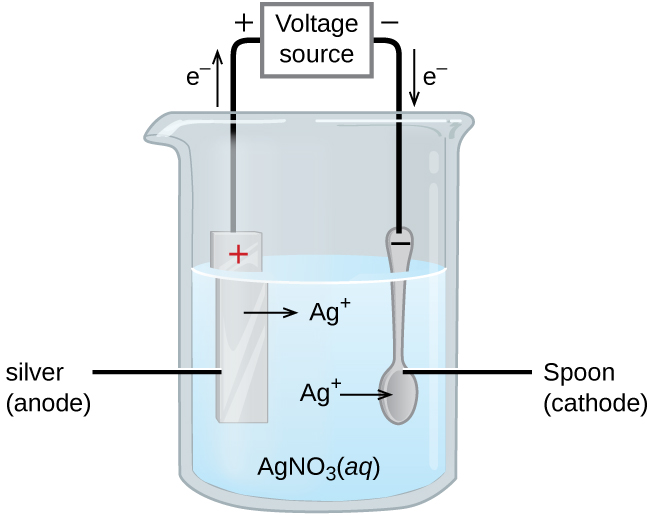
Comments
Post a Comment